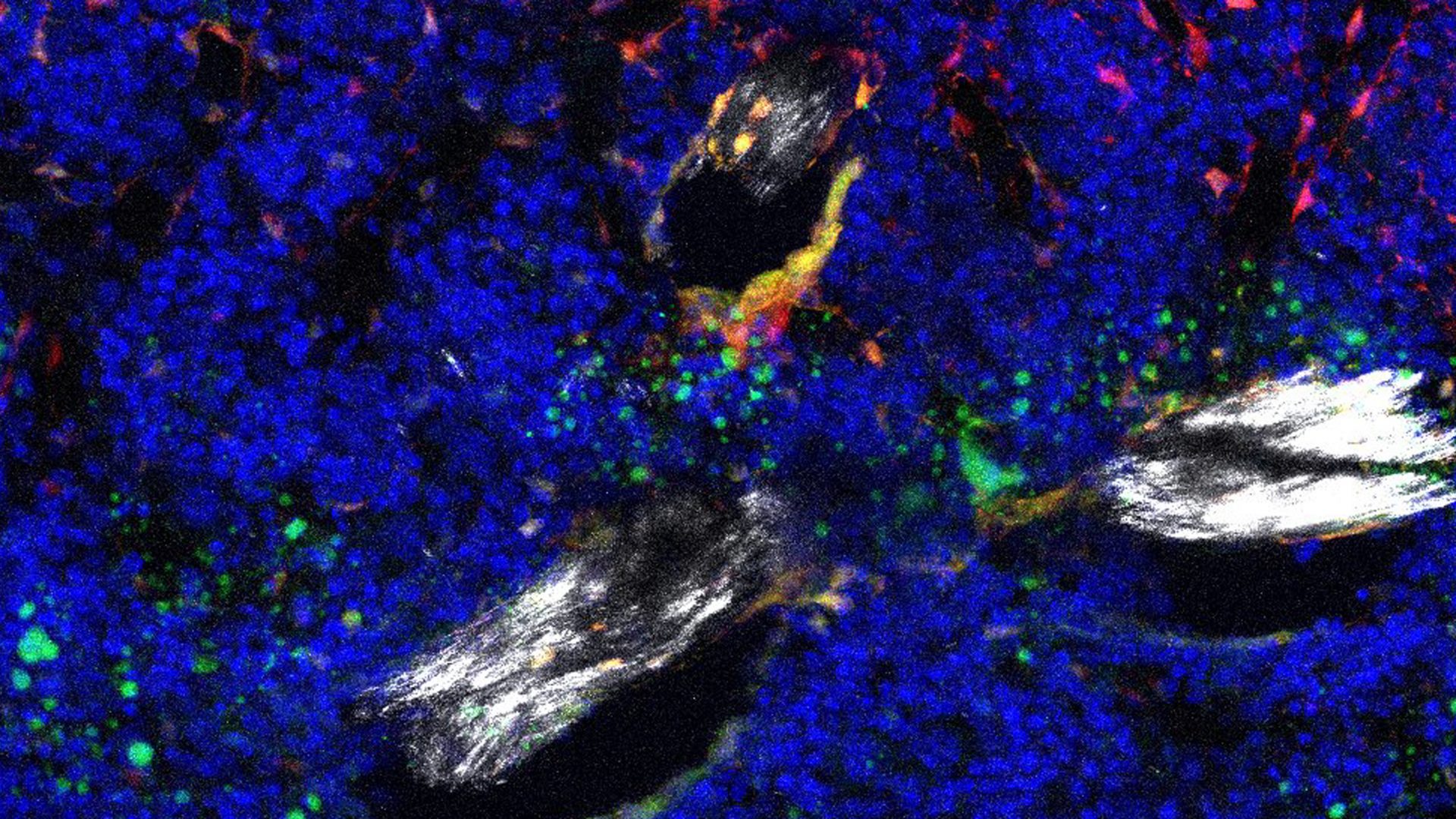
Single-cell RNA sequencing of young and aged mouse hindlimbs points to an abnormal increase in Notch signaling in skeletal stem cells as playing a central role in age-related bone loss, according to a recent report in Bone Research.
Findings from the study suggest that interfering with the signaling pathway by targeting a protein called Ebf3/EBF3 has therapeutic potential in preserving skeletal integrity and preventing deterioration.
“Our findings reveal that Notch in skeletal stem cells becomes abnormal with age and that blocking it prevents age-related skeletal degeneration.”
Philipp Leucht, MD, PhD
“Our findings reveal that Notch in skeletal stem cells becomes abnormal with age and that blocking it prevents age-related skeletal degeneration,” says senior study author Philipp Leucht, MD, PhD, an orthopedic surgeon at NYU Langone Health.
The Bone-to-Fat Shift
Bone is among the tissues that keep self-renewing pools of stem cells, called skeletal stem and progenitor cells (SSCPs), on hand into adulthood.
SSCPs can mature into replacement cells that maintain healthy tissue and heal broken bones, with maturation following one of two paths: new bone-making cells called osteoblasts or fat-making cells called adipocytes.
Past studies have shown a notable, gradual shift in the fate of SSCPs during aging. Instead of becoming osteoblasts, there is a tendency toward differentiation into adipocytes as SSCPs age. This alteration in cell fate leads to several consequences, including bone loss and increased adipose tissue within the bone marrow. Consequently, bones become weaker and more vulnerable to fractures.
“The reprogramming of adult stem cells as a source of bone-making cells in healing-compromised people has profound therapeutic potential.”
Currently, there are no therapeutics to rescue or maintain the bone-making function in aging SSPCs. Clarifying the mechanisms that trigger the bone-to-fat shift may serve to “prolong healthspan, preserve skeletal integrity, and enhance the efficiency of fracture healing in the aging population,” write the study authors.
Notch Activity and Stem Cell Fate
As reported in the new study, increased Notch pathway activity appears to shift SSCPs toward the fate that increases fatty degeneration of bone marrow.
When the authors genetically engineered mice to lack Nicastrin, a core part of the Notch signaling pathway, SCCPs were redirected toward differentiation into osteoclasts, increasing bone formation “even beyond that of young mice,” write the authors.
Other effects included a specific increase in trabecular bone formation, reduction in bone marrow adiposity, and enhanced regeneration, which the authors posit could curtail skeletal degeneration.
Identifying a Potential Therapeutic Target
Notably, Notch signaling, woven as it is into so many cellular pathways, is not a good target for drug therapy, Dr. Leucht stresses. Instead, his team identified Ebf3 as a promising potential therapeutic target, confirming that the transcription factor carries the Notch signal forward and is relatively restricted to being made in skeletal stem cells.
“The reprogramming of adult stem cells as a source of bone-making cells in healing-compromised people has profound therapeutic potential. In future studies we hope to confirm the value of Ebf3 as a drug target in preventing osteoporosis,” says Dr. Leucht.
Notch signaling is important to the development of many tissues in the womb but is then suppressed to enable healthy adult tissue function, Dr. Leucht adds. Diseases like cancer—and the aging process—are linked to the abnormal reactivation of developmental processes, where the genetic systems (heterochromatin) that block access to certain genes break down. Thus, the current study’s finding that Notch-related genes were abnormally upregulated could reveal disease mechanisms behind forms of deterioration seen in other tissues and organs.